Summary
1. What is EUDAMED?2. A multi-purpose and interoperable system
3. Is EUDAMED already in use?
4. In conclusion
What is EUDAMED?
EUDAMED (European database on medical devices) is a system that is a European-wide multi-purpose and interoperable medical device database. It is a collaborative system for recording, reporting and dissemination of information available in part to the public. All with the aim of making more transparent and improving information sharing on medical devices at the European level.
A multi-purpose and interoperable system
We have seen that the EUDAMED database is characterized by being multi-purpose and interoperable. But what exactly are we referring to by these two terms?
Interoperability comes from the fact that different actors can interact with the platform by uploading the information required by European regulations. We are referring, in this case, to the actors defined in the MDR (such as manufacturer, authorized representative, importer and distributor) and Notified Institutions. However, dentists, dental technicians and patients themselves can also access these data for consultation.
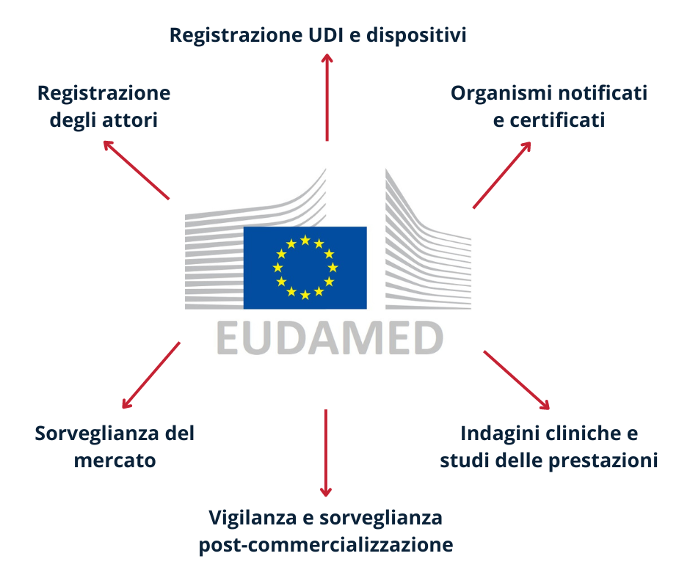
On the other hand, as far as multi-purpose is concerned, reference should be made to the final configuration of the database, which will consist of a single website, which links to six different electronic systems (so-called “modules”) interconnected with each other:
Interoperability comes from the fact that different actors can interact with the platform by uploading the information required by European regulations. We are referring, in this case, to the actors defined in the MDR (such as manufacturer, authorized representative, importer and distributor) and Notified Institutions. However, dentists, dental technicians and patients themselves can also access these data for consultation.
On the other hand, as far as multi-purpose is concerned, reference should be made to the final configuration of the database, which will consist of a single website, which links to six different electronic systems (so-called “modules”) interconnected with each other:
- actor registration;
- UDI registration and devices;
- notified bodies and certificates;
- clinical investigations and performance studies;
- post-market surveillance and surveillance;
- market surveillance.
Is EUDAMED already in use?
It is important to note that the full operability of EUDAMED has not yet been achieved, nor has its mandatory nature.
Of the six modules that make up the system, only three are currently operational: the module on the registration of actors, the module on the registration of medical devices and their UDIs, and the module for Notified Bodies.
Only when the system is fully functional and notice of full functionality to be published in the Official Gazette is given, Article 34 paragraph 3 of the MDR establishes a 24-month period within which all obligations for registration of medical devices and certificates must be completed.
Of the six modules that make up the system, only three are currently operational: the module on the registration of actors, the module on the registration of medical devices and their UDIs, and the module for Notified Bodies.
Only when the system is fully functional and notice of full functionality to be published in the Official Gazette is given, Article 34 paragraph 3 of the MDR establishes a 24-month period within which all obligations for registration of medical devices and certificates must be completed.
In conclusion
To date, therefore, EUDAMED presents a purely voluntary functionality and use. Nonetheless, BioService, always attentive to the needs of its clients and the obligations imposed for better transparency of medical devices, has already taken care of its own registration as a manufacturer, and will also soon complete the extensive requirements of the second module.
Keep following us so you don't miss updates regarding EU Regulation 2017/745 (MDR) and the latest BioService Corporate Quality news!
Keep following us so you don't miss updates regarding EU Regulation 2017/745 (MDR) and the latest BioService Corporate Quality news!

